The efficient and safe healthcare relies on complete traceability of pharmaceutical products. Accurate tracking, from suppliers of medical components and drug manufacturers through administration and disposal, ensures operational efficiency and improves patient safety. However, traceability is not only key for minimising risks within medical supply chains, but also for managing medical waste. Without proper traceability, expired medications and medical supplies often go unnoticed until they can no longer be used, leading to unnecessary financial and environmental burdens. Although calculating precise quantification of wasted medicine costs is complex, in the UK it is estimated that between £200m and £300m of unused or partially-used medications are discarded each year.
Serialisation offers a wide range of traceability advantages by assigning unique digital identifiers to products and thereby enhancing transparency, accountability and control throughout the supply chain. Its effectiveness in improving medical procedures, though, relies on the underlying systems and technologies.
Enhancing traceability
Ensuring the traceability of primary containers, especially prefilled syringes (PFS), presents unique production, handling and compliance challenges compared to traditional vial and syringe systems. PFS’s popularity is driven by its ease of use, accurate dosing and increased safety, yet these advantages necessitate stronger traceability and integration from pharmaceutical manufacturers and distributors to medical practitioners and regulatory authorities. Each individual syringe must be linked to its corresponding batch records during production, monitored for storage conditions throughout transportation, and then traced through to the moment of administration.
Poorly managed PFS tracking can result in unnecessary medical waste and potentially compromise patient safety. Furthermore, robust supply chain traceability can help to increase the difficulty of counterfeit drugs entering supply chains. The EU loses an estimated €950m annually due to counterfeit medicines, according to the European Commission. Identification and tracking at every stage of distribution, authentication of each package ensures that only genuine medications traverse the supply chain, while precise tracking deters theft or diversion into unauthorized markets.
Nevertheless, the success of supply chain traceability depends on the scope of its deployment, and batch-level identification can lack granularity, impacting the effectiveness of traceability measures. For example, individual serialisation can also help to aid quality control within laboratories and production lines where temperature control is critical. Biologics, vaccines and specialty medications often require continuous refrigeration from the moment of manufacture until the point of administration. Even short periods outside the allowed temperature range can compromise potency and stability, leading to unsafe batches that must be discarded. The inability to track individual syringes means that any temperature issue impacts the entire batch, requiring its disposal.
With individual serialisation, it is possible to only flag non-compliant syringes, improving efficiency and reducing waste. Until recently, pharmaceutical companies largely relied on barcodes to track primary and secondary medical packaging. In these conventional systems, barcodes affixed to cartons or individual syringes must be oriented precisely beneath scanners, and any deviation in label placement or surface damage can result in missed reads.
Furthermore, the need for line‑of‑sight scanning introduces delays and human error during high‑volume inspections. While barcode‑based serialisation has undoubtedly improved medical traceability, its methods struggle to provide the unit‑level precision and real‑time integration that modern healthcare demands.
RFID technology for medical traceability
RFID is increasingly seen as a cornerstone technology in digital health ecosystems, interfacing with hospital information systems (HIS), enterprise resource planning (ERP) tools, and logistics automation platforms. Murata’s RFID solution offers a groundbreaking approach to the challenges of production integrity, high-speed throughput and global interoperability.
Murata’s class-smallest RFID tag integrates both the microchip and its antenna into a ultra-thin assembly that can be embedded directly into syringe components during manufacture. This highly compact system is designed for prefilled syringes, vials and cartridges, and integrates into product caps, rigid needle shields (RNS), and connection mechanisms like Luer Lock systems, enabling full lifecycle tracking from production to end use. Murata’s method is designed to improve workflow efficiency, ensures clear visual inspections, and helps to lower risks associated with drug administration.
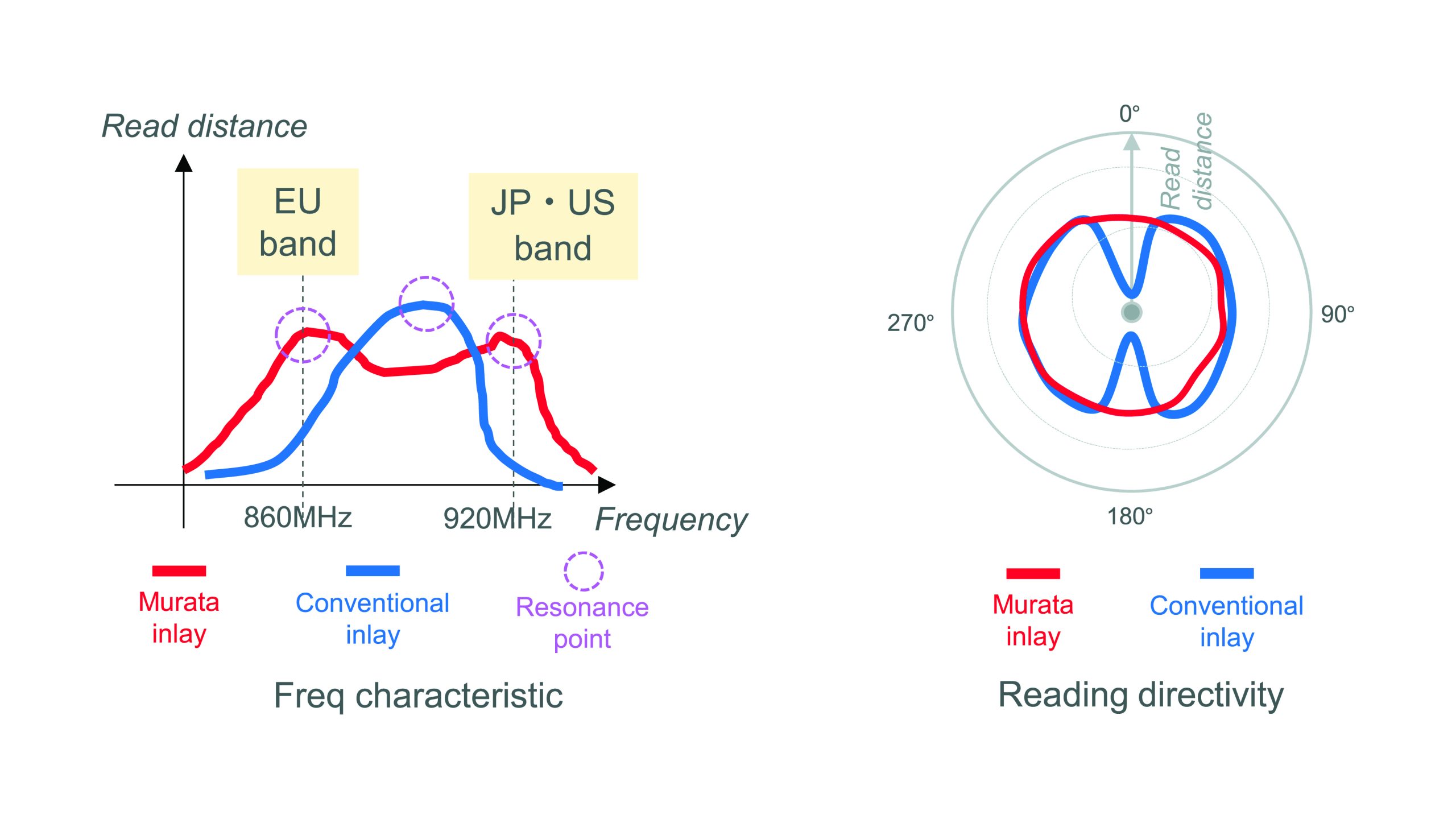
As well as the RFID tag, Murata’s technology can be packaged in a 20mm x 5mm inlay. Powered by Murata’s coupling module technology, this inlay features a matching and resonance circuit, and its patented wraparound antenna design offers full 360° readability, eliminating dead zones found in conventional inlays.
Murata’s RFID technology is designed to support high throughput manufacturing settings, facilitating the seamless identification of over 600 units per minute from a single dynamic reading. Each antenna is a dual-resonance design, enabling operation within the European 860MHz band and the 920MHz band used in the US and Japan; see figure. Consequently, using these RFID tags eliminates the need for region-specific SKUs, thus allowing a single, universally readable syringe to be used globally, simplifying inventory management and accelerating the time to market.
Every tag offers item-specific serialisation, enabling precise tracking of individual syringes, vials, or cartridges, and facilitating batch separation if a post-market defect arises. RFID tags present a more efficient and reliable solution compared to standard QR codes, as the latter require a clear line of sight for scanning and may interfere with PFS’s visual inspections.
Embedding the Unique Device Identification (UDI) directly into the RNS or Luer Lock system allows direct access and updates of product information from an Enterprise Resource Planning (ERP) system, minimising manual data entry errors and eliminating potentially contaminating retrofit labels. This immediate data integration improves the digital chain of custody while meeting strict regulations such as FDA 21 CFR Part 11 and the EU Falsified Medicines Directive without sacrificing syringe sterility or ergonomics.
RFID in action
When embedded into pharmaceutical workflows, Murata’s RFID technology is designed to provide a wide range of benefits at each stage of the product lifecycle.
Case Study 1: Incoming Inspection of Prefilled Syringes
When prefilled syringes arrive at a pharmaceutical facility, they must undergo rigorous inspection to ensure that each unit meets stringent quality standards before proceeding to the filling line. Traditionally, this process has involved manual sampling of a small proportion of the shipment and time-intensive visual checks, both of which can delay production and introduce human error. By contrast, Murata’s RFID solution assigns each syringe a unique digital identity that is read automatically as it passes through an RFID gateway. Any unit that deviates from its quality control record is immediately diverted into quarantine, eliminating the need for routine manual sampling and accelerating throughput.
Each RFID read can be logged in a centralised digital ledger, capturing the UDI, batch number, and relevant QC data for every syringe. This comprehensive audit trail underpins compliance with Good Manufacturing Practice (GMP) standards, ISO 11040 guidelines, and regulations such as FDA 21 CFR 610.14 and the EU Falsified Medicines Directive. This pre-validation capability also enables automated rejection of full batches where manufacturer-supplied QC data indicates systemic issues. As a result, manufacturers can benefit from fewer line stoppages and reduced rework, while real-time visibility into inventory status drives down material waste and yields significant cost savings.
Case Study 2: Monitoring Excursion Time
Time Out of Refrigeration (ToR) is the duration for which a temperature-sensitive product exceeds its prescribed cold-chain range, and is a critical metric for biologics, vaccines, and specialty medications. In conventional workflows, a single batch-level temperature excursion often mandates the disposal of the entire lot. By contrast, Murata’s RFID tags allow for unit-level ToR data to be recorded with every scan.
Should a syringe exceed its allowable ToR threshold, only that unit is flagged and removed, while compliant items continue through the process. This precision can significantly reduce physical and monetary waste, preserving supplies for urgent clinical needs. With high-value products, this can translate into substantial cost avoidance at the pallet level. Moreover, aggregated ToR logs can become a diagnostic resource, enabling quality teams to analyze excursion patterns, identify handling or storage inefficiencies, and implement targeted corrective actions, whether by refining standard operating procedures or upgrading refrigeration equipment.
Case Study 3: Aftermarket Applications
Murata’s solution also has the potential to extend beyond the production of primary and secondary containers into connected device and aftermarket ecosystems. In smart, rechargeable insulin injectors, for example, embedded RFID tags can relay usage data to cloud platforms, enabling real-time patient monitoring and adherence tracking. Similarly, the same tags can store links to digital instructions or batch specific documentation, bolstering counterfeit prevention and enhancing end user confidence. By uniting hardware miniaturization, advanced RF design and seamless data integration, Murata’s RFID technology is designed to provide medical professionals with a level of traceability and operational efficiency that was previously unattainable.
Data-driven healthcare with embedded RFID
As healthcare systems seek to balance efficiency, safety and sustainability, serialisation technologies are becoming indispensable. Murata’s embedded RFID solutions are designed to directly address the limitations of conventional barcode and QR systems, enabling unit-level traceability that scales across global manufacturing and supply chains.
Moreover, the integration of Murata’s RFID into syringe components supports automation, reduces human error, and opens new frontiers in smart device connectivity and aftermarket applications, especially as personalized medicine and biologics continue to grow. By providing pharmaceutical stakeholders with instant access to product-level data, from production through inspection, storage, and administration, Murata’s technology is helping medical professionals to mitigate costly inefficiencies and enhance patient safety.
By Kenya Watanabe, Product Manager, Murata